Plastic as a problem is no longer a new phenomenon, with ever-growing focus and concern on the volume of plastic in the environment. According to Greenpeace(1), a truckload of plastic enters the ocean every single minute, and UK supermarkets produce 800,000 tonnes every year. With the world’s sights focused on this problem, it becomes more important that laboratories and scientific institutions are producing robust and useful data.

What are microplastics?
Less than 5mm in diameter, microplastics are fragments or particles of plastics from macroplastic sources that have reduced in size. Plastic carrier bags, bottles, packaging and straws are just a few examples of plastic sources that can reduce down to microplastic particles, as well as microfibres from synthetic clothing. Microbeads, an intentionally created microplastic for the benefit of the cosmetic industry, have also added to the plastic pollution of the environment.
Although anything less than 5mm is classified as microplastic, there is no definitive minimum size before it is classified as a nanoplastic. Nanoplastics are plastic fragments thousands of times smaller than microplastics, which could have an effect on organisms by being absorbed into the body and passing through into cells. Once inside the cell, it has the potential to cause physical damage from the fragment structure, or from the chemicals that could be absorbed within the nanoparticle.
How to analyse a land sample and issues faced by the laboratory
There have been various samples analysed for microplastics such as biota, water and air. One area that can cause challenges is the examination of land samples, such as soils, landfill and composting material. This is due to the significant amount of non-plastic particles present in such samples – for example, sand, stone, coal, metal and plant material.
The first thing to decide upon is the size range of microplastics to be isolated. Wet sieving to remove the larger and smaller particle range will help to isolate the fraction for analysis. This fraction, which will contain the microplastics of interest, is then subjected to a density flotation and organic digestion.
Plastics have a density ranging between 0.91g/cm3 for low density Polyethylene up to 1.38g/cm3 for Polyethylene terephthalate. This density range of microplastic particles will need to be floated, therefore a flotation liquid of at least 1.4g/ml would be suitable to be employed. The use of a saturated Sodium chloride solution (density 1.2g/ml) should be avoided, as heavier plastics such as the Polyethylene terephthalate and Polyvinyl chloride would be lost.
A suitable flotation liquid to use would be a solution of sodium heteropolytungstates, which can be made to a density of up to 2.8g/ml. A custom modified flotation vessel made from a separating funnel is shown in figure 1. The floated solids will now contain the plastics, along with impurities such as organic material and lighter coal particles. To remove the organic material, a digestion step is employed.

Digestion can be performed in a number of ways, from using a complex enzymic digestion to a simpler Hydrogen peroxide digestion. Either technique will break down the organic material over a period of hours to days and does not damage the plastic particles. Samples that have a high level of organics, such as compost, will take a significant digestion process.
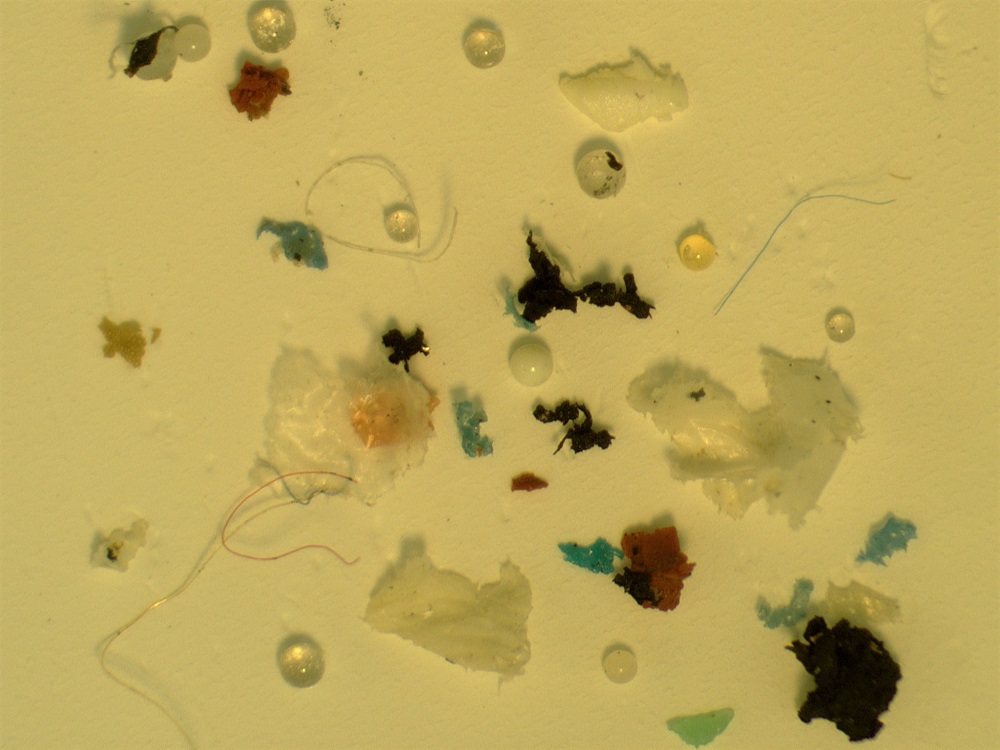
Post-digestion, the residue is washed and filtered ready for analysis to determine the microplastics. High levels of coal and some fly ash compositions can cause issues, as they can have the density within the plastic range and are unaffected by the digestion process. This means that they will be seen in the fractions sent for analysis. Figure 2 shows an example of recovered microplastics from a river sediment sample – you can see a variety of colours, sizes and shapes.
Keeping a control on quality
Quality control is critical in any analysis. The two main considerations we should take into account are the control of contamination and the effectiveness of the microplastic isolation.
Microplastic contamination is a constant issue that the laboratory must be aware of and mitigate its effect. Microplastics are present in dust and fibres from clothing floating in the air. It is critical that a system blank is performed with samples to prove that contamination is controlled and at an acceptable level. Working in laminar flow cabinets and keeping vessels covered in tinfoil can help with keeping samples protected from the environment. In addition, any potential contamination from plastics and rubbers that form part of the extraction process (such as vacuum tubing or seals) should be considered.
Assessing how well a preparation technique is at recovering the microplastics of interest can be a challenge due to little Certified Reference Material (CRM) being readily available. The ability to check recovery and the production of a laboratory-produced CRM is something to be considered.
A mix of waste plastics covering the range of polymer types can be freeze dried with liquid nitrogen and ground to produce a ‘microplastic mother sample’. This mother sample can be spiked into a matrix (such as a cleaned furnace sand) by either weight or number count. Microplastics standards are now also starting to become commercially available, with vials of microplastic mixes that you can spike into samples or use as calibrations. When you know what you have added in, you can determine a percentage recovery.
Analytical techniques
When you have isolated your microplastics, you have to do some analysis to determine what you have. There are a number of techniques ranging in price, technical ability, time of analysis and the types of results obtained.
Microscopy
Optical microscopy is the simplest and lowest cost technique available, although it is physically time consuming. Under the microscope, we can identify the presence of a plastic by its physical characteristics. Care should be taken, especially so that any undigested natural fibres are not misidentified. From the microscope image, we can count the number of microplastics and identify their colour, shape and size. Commonly, microplastics are grouped as fibres, spheres and fragments. Optically, it is challenging to investigate particles below 100um.
Two techniques that can be used with microscopy to help identify the presence of plastic is the hot needle and Nile Red dye. The hot needle technique is where you touch the particle with a heated metal needle under the microscope and watch to see if the particle melts. If it melts, this then indicates that the particle is plastic.
The Nile Red dying technique(2) involves incubating the sample for 30-60 minutes with a solution of the phenoxazone lipophilic fluorescent dye ‘Nile Red’. This is followed by excitation under a blue light (Crime Lite: 450-510nm) through an orange filter (529nm) which results in the dyed plastics fluorescing, making it easier to positively identify the fragment as being of plastic origin.

FTIR vs. Raman Spectroscopy
Fourier Transform Infrared (FTIR) and Raman spectroscopy are analytical techniques that can identify the polymer type of the microplastic particle. Both techniques acquire a spectral fingerprint that we can compare against a library of polymers to match the unknown particle. FTIR spectroscopy is where mid-infrared light is passed through the sample. Particular molecular bonds present will absorb specific amounts of energy, and these corresponding losses of energy are recorded and produce a peak.
Raman Spectroscopy is a vibrational spectroscopy technique where a single wavelength of incident light from a laser is directed onto the sample. The laser excites the bonds of a molecule, which generates scattered light that identifies the bond in question and produces a fingerprint we can again search against a library to identify the polymer.
Both FTIR and Raman spectroscopy are capable of identifying the polymer of the microplastic. Raman spectroscopy does have an advantage over FTIR in relation to microplastic size. Raman uses a sub-micron wavelength laser as the light source and is capable of resolving particles down to sub-micron size. FTIR microscopy uses a mid-infrared light as the source, resulting in a lower resolving power and particles below 10 micron in size. Cost also plays a part, as FTIR spectroscopy is much more common (the price is less than a Raman spectroscopy system).
Although both systems can identify the polymer type, the Raman spectroscopy does suffer from fluorescence interference. Some samples can exhibit fluorescence when irradiated by the laser, which can then interfere with the readings. This is not a problem seen with FTIR spectroscopy. A combined approach uses larger microplastics by FTIR spectroscopy and the sub 10-micron size by Raman spectroscopy. Whichever spectroscopy technique is used, the sample must be introduced to the instrument. The two main techniques for microplastics are Attenuated Total Reflection (ATR) accessories and microscopes.
An ATR sample measurement involves placing a single microplastic onto the viewing crystal (usually a diamond), locking it down with an anvil to ensure good surface contact. The infrared light is reflected off the surface of what is pressed onto the diamond and into the detector. Although the ATR technique is a cost-effective and easily introduced technique, its limitations are that it is a manual preparation and is limited by the size of particle that can be introduced (~5mm to 50um).
The second main technique is the FTIR and Raman Microscopes. These techniques allow for a more precise pinpointed analysis of the smaller microplastics that are difficult to manually manipulate. The isolated microplastics are filtered down to allow direct spectroscopic analysis on filters such as silicon or gold-coated polycarbonate. Under the microscope, the particles can both be manually picked and scanned. Alternatively, by using an imaging microscope system, the whole surface is scanned and the IR/Raman spectrum is taken for each pixel. This allows automated systems to be employed to identify different polymers and the software to report types of polymer, counts and sizes of the individual particles.
Raman spectroscopy uses sub-micron wavelength lasers on the microscopes, which is capable of resolving particles down to 1 micron, whereas FTIR microscopes use mid-infrared light, which limits its capacity to 10 microns. The analysis technique for FTIR microscopy must also be considered, as well as transmission or reflectance. Transmission is where the light passes through the sample, which tends to give a better spectrum. However, the particle is required to be less than 100 microns thick to allow the infrared light to pass through. Reflectance is where the light is reflected from the surface of the particle, which means thickness is not an issue. However, the spectrum obtained can be weaker and distorted, making it more difficult for identification.
With any of these spectroscopy techniques, identification of the polymer particle can be challenging. All spectra will need to be searched against a library of known polymer spectra. Care should be taken when comparing to pure spectra libraries, as these libraries are produced from clean polymers. The polymers seen from microplastics could have been in the environment for a number of years and have been subjected to weathering and UV degradation. This can make identification against clean polymer libraries more challenging. Building up a custom library in the laboratory of weathered/degraded plastics can help significantly with the identification of real-life microplastics.
Thermal analysis
Pyrolysis Gas Chromatography Mass Spectrometry (Py-GC-MS) is a thermal technique that is gaining popularity in the analysis of the isolated microplastic fraction. As with all techniques, an isolation procedure is still required to be carried out. The final purification is not as important, as materials such as stone and coal will not interfere with the analysis. The final fraction containing the microplastics are transferred to a combustion cup and introduced to the pyrolyser, where it is subjected to high temperatures (600oC). The resulting pyrolysis gases are transferred to the GC column under a stream of Helium.
The individual pyrolysis gases are chromatographed out on the GC analytical column before being detected by the mass spectrometer. Each polymer type will produce different pyrolysis products – for example, Polyethylene producing alkanes/alkenes and Polystyrene producing Styrene. The presence of these marker products allows for the identification of the polymer, and the response will quantify the mass of that polymer present. Calibrations are performed by known masses of polymers analysed under the same conditions as the samples, producing calibration curves for accurate quantification.
There are a number of advantages to using this technique. Analysis can be automated by loading the combustion cups containing the isolated microplastic samples onto an autosampler. This will take each sample in turn and analyse, allowing for analyst free analysis. The mass spectrometer is a highly sensitive technique and can detect down to nanogram levels. The biggest disadvantage with thermal analysis is that although it will identify the polymer present and calculate the mass, it will give no indications on particle size, shape or number. It is also a destructive test, so no sample will remain after combustion to allow for any further analysis.
What is next on the horizon?
As we have seen, there are many different techniques for the analysis of microplastic in environmental samples, be it water, soils, sediments, biota or air. Different techniques will give varying results – this is based on the size of microplastic able to be isolated for analysis, which will determine whether the technique of choice will identify the number, shape, size, colour or mass. With such a varying array of potential results, it makes it extremely difficult for the scientific community to compare results.
Certainly, some kind of standardisation must soon come into effect. Committees led by such institutions as British Standards, ASTM and EPA are currently investigating standardised methods for the sampling and analysis of microplastics. The methods that are developed will need to be robust and suitable for laboratories to implement and provide the required data format that is requested by governing bodies. Until legislation controls and limits are put into place for microplastic amounts in the environment, it is difficult to know what analytical technique will be required.
One question that needs answering is what health and environmental effects microplastics actually cause the planet and its inhabitants. Is it the plastic type, shape, size or mass that is the issue? The answer to that will determine what analytical technique will need to be used. The smaller the microplastic, the more challenging it is to analyse, especially in such a complex matrix as soil and sediment. Certainly, nanoplastics (particles in the namometer range) have the potential when ingested into the body to cross membranes into cells, and when airborne, can be inhaled and deposited in airways and lungs.
The larger microplastics could still be an issue, as they could be acting as a carrier of toxic materials such as heavy metals and Persistent Organic Pollutants (POPs) into the body. POPs are chemicals that persist in the environment, bio accumulate through the food web and pose a risk of causing adverse effects to human health and the environment(3). POPs include chemicals such as Pesticides, Polychlorinated biphenyls and Dioxins. Exposure to these chemicals can cause developmental problems, chronic illnesses and death.
Research into the actual health effects of microplastics is ongoing around the world, and results are eagerly awaited. Although microplastic analysis is certainly challenging, a standardised approach to provide the data in a useable form will no doubt give the scientific community the results required to monitor the prevalence and effect on our environment.
- https://www.greenpeace.org.uk/challenges/plastic-pollution/
- Maes, T., Jessop, R., Wellner, N. et al. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci Rep 7, 44501 (2017). https://doi.org/10.1038/srep44501
- https://ec.europa.eu/environment/chemicals/international_conventions/index_en.htm.
About SOCOTEC UK
In the UK, SOCOTEC is the leading provider of testing, inspection and compliance services, offering comprehensive solutions for the Infrastructure & Waste and Environment & Safety sectors. The company, which employs more than 1,700 people, delivers in excess of seven million tests a year to over 5,000 customers.
With a history dating back over 100 years, SOCOTEC UK has nationwide coverage on client sites and a network of 20 UKAS-accredited laboratories, and continues to be a significant driver of change and innovation within the industry. By helping clients design solutions and remain compliant to changing legislation, international standards and economic conditions, SOCOTEC has become the UK’s leading partner in its chosen sectors for technical expertise and services.
Author
Paul Walker (BSc, MRSC) gained his Chemistry degree from Grey College, Durham University, and has worked for SOCOTEC UK for 25 years. As senior technical and development specialist in the Environmental Science sector at SOCOTEC UK, Paul is responsible for developing analytical methods, both for improving current methods and implementing new work areas. Paul is an expert in the field of environmental forensics and currently is involved in the examination of soils and sediments for microplastics.
Want to find out more about how SOCOTEC UK can support your organisation?

You might also like







Add new comment